CCUS (Carbon Capture, Utilization, and Storage) is a rapidly emerging industry poised to play a critical role in companies' decarbonization strategies. According to global growth consultancy Frost & Sullivan, the CCUS market is expected to grow at a compound annual growth rate (CAGR) of 49.7% between 2022 and 2030. By 2030, the market revenue is projected to reach $42.48 billion, and by 2034, it could peak at $45.21 billion.
The utilization of captured CO2 for high-value products is a key step in enhancing the value of CO2. Polymers, being high-value products, can be synthesized from CO2 through catalytic or other processes, adding value to the CO2 supply chain and creating environmentally sustainable pathways.
The following diagram summarizes some of the more promising CO2-to-polymer conversion routes:
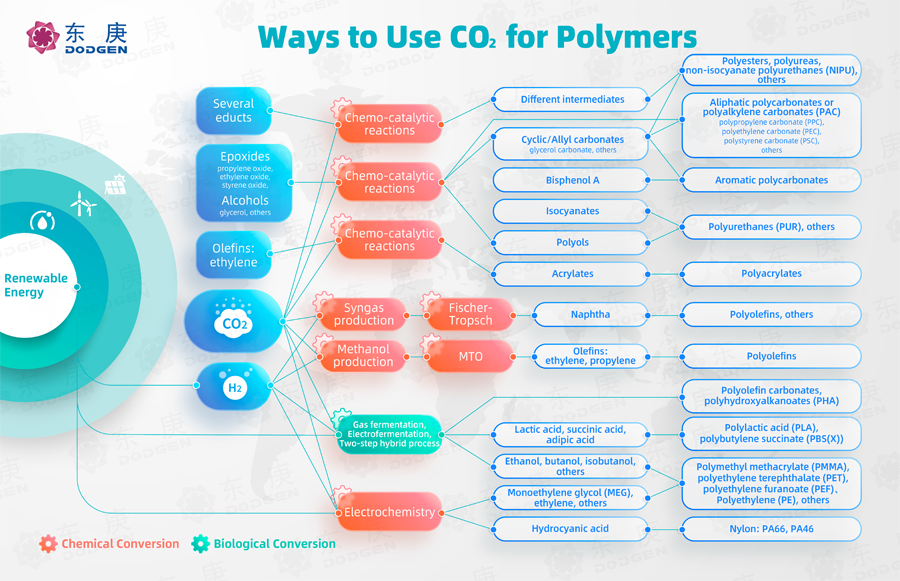
I. Key Route Overview
Catalytic Conversion
Catalysis of CO2 with extracts generates intermediates that can eventually form polymers such as polyesters, polyureas, and non-isocyanate polyurethanes (NIPU).
CO2 and epoxides (e.g., ethylene oxide, propylene oxide) undergo polymerization reactions in the presence of catalysts, resulting in aliphatic polycarbonates or polyalkylene carbonates (PAC).
CO2 with epoxides or alcohols catalyzes the synthesis of polyols, which can then combine with isocyanates to form polyurethanes.
Catalytic conversion of CO2 and H2 into syngas, followed by Fischer-Tropsch reaction, leads to polyolefins. Polyolefins are widely used polymers, including polyethylene (PE) and polypropylene.
Hydrogenation Route
CO2 and hydrogen (H2) react through a series of hydrogenation steps to produce methanol, which can then be converted into olefins (e.g., ethylene, propylene) through methanol-to-olefins (MTO) technology, leading to polyolefin synthesis.
CO2 and hydrogen (H2) through electrochemical reactions can produce ethylene glycol (MEG), ethylene, and hydrocyanic acid, which can further be synthesized into polymers like polymethyl methacrylate (PMMA), polyethylene terephthalate (PET), polyethylene furanoate (PEF), and polyethylene (PE). This route is efficient, environmentally friendly, and an important future direction for CO2-to-polymer conversion.
Fermentation
CO2 and green hydrogen through fermentation can produce intermediates such as lactic acid, succinic acid, adipic acid, ethanol, butanol, isobutanol, and others, which can be further synthesized into biodegradable biopolymers like polyhydroxyalkanoates (PHA), polylactic acid (PLA), and polybutylene succinate (PBS). These materials hold broad application prospects.
These summarized routes demonstrate multiple possibilities for converting CO2 into polymers. These pathways are not only theoretically feasible but have also made some progress in experimental research and industrial application. However, in practical industrialization, factors such as technology maturity, economic feasibility, market demand, and environmental impact need to be considered. In the future, as technology advances and costs decrease, these routes could become important pathways for CO2 resource utilization.
II. Technology Maturity of Key Routes
Certain routes, such as the production of polyesters, polyureas, and non-isocyanate polyurethanes (NIPU), have relatively mature technologies, but the direct conversion rates from CO2 may still be limited.
Polycarbonate polymer production (e.g., polypropylene carbonate (PPC), polyethylene carbonate (PEC)) is rapidly developing, though further research and optimization are needed to improve yields and reduce costs.
Electrochemical and Fischer-Tropsch technologies show promise but are currently at the laboratory or small-scale industrial testing stages, requiring further R&D investment and validation.
CO2-to-polyol technologies have achieved some industrial application success. For example, some companies have developed complete sets of proprietary technologies for CO2-derived polyols, ranging from catalysts, reaction processes, and equipment to downstream applications, widely used in polyurethanes, synthetic leather, and foams. As technology progresses, CO2-derived polyols may see broader applications and greater development.
III. Raw Material Costs
CO2 as a raw material has the advantage of being low-cost, as it is a widely emitted greenhouse gas that can be captured and utilized. However, the costs of other auxiliary raw materials (e.g., hydrogen, catalysts, solvents, biomass) may vary depending on their source, price, and market supply.
For fermentation-produced polymers (e.g., polylactic acid (PLA), polyhydroxyalkanoates (PHA)), the raw material costs (e.g., sugars, biomass) and fermentation process efficiency also impact economic feasibility.
IV. Market Demand
The size and growth rate of market demand will directly affect the economic feasibility of these technologies:
With increasing environmental awareness and the demand for sustainable development, there is a growing demand for biobased and biodegradable polymers, which helps drive the development of CO2-to-polymer conversion technologies.
As the potential for low-carbon fuels becomes apparent and the global push for sustainable development continues, the market demand for green methanol is steadily growing. Green methanol has extensive applications, including automotive fuel, fuel cells, marine fuel, and organic additives. As CO2 conversion and green hydrogen technologies progress and costs decrease, green methanol will see increasing application in these fields, with sustained market demand growth.
V. Environmental Impact
CO2-to-polymer conversion technologies help reduce greenhouse gas emissions, plastic pollution, and promote resource recycling while minimizing waste generation. However, some routes may produce other pollutants or have potential environmental impacts.
A comprehensive environmental impact assessment is needed, along with appropriate measures to mitigate negative effects.
Despite the many environmental benefits of CO2-to-polymer conversion technologies, they still face technical challenges. For instance, improving CO2 conversion efficiency and optimizing polymer performance to meet different application needs are key areas of focus. However, with continuous efforts from research institutions and companies, these technical challenges are gradually being addressed. In the future, as technology continues to advance and applications expand, CO2 conversion technologies will play an increasingly important role in environmental protection and resource recycling.
Conclusion
In conclusion, the outlined CO2-to-polymer conversion routes present various challenges and opportunities in terms of technical feasibility and economic viability. To achieve the commercialization of these technologies, further R&D is required to optimize process conditions, reduce production costs, and improve energy efficiency, while considering market demand and environmental impacts. Additionally, government policies, funding support, and market demand will play significant roles in shaping the development of these technologies.