Extraction methods separate mixtures based on the difference in solubility of different substances in immiscible solvents. The extraction and crystallisation process can be seen as a result of the coupling of ‘extraction’ and ‘crystallisation’. The principle can be divided into two categories:
(1) the force between the extractant and the solvent is greater than the force between the target product and the solvent. The combination of extractant and solvent reduces the solubility of the target product, which then precipitates by crystallisation.
(2) Melt crystallisation technique utilises the difference in freezing points between the components of a substance to achieve separation and purification. By controlling the input and removal of heat, the separated components are made to crystallise and precipitate from the molten liquid, and then the target components are separated and purified by washing, sweating and other operations.
The coupled melt crystallization process and extraction combines the advantages of both, initially separating the target substance through the extraction process and then further purifying it through melt crystallisation.
Many researchers have used the coupled process of melt crystallisation and extraction to separate and purify substances with good results.
In the study of the extraction-coupled melt crystallisation process of 2,6-dimethylnaphthalene from Ø coal tar, the process usually includes the following steps:
1. Pretreatment: The coal tar is pretreated by technology distillation to separate the fraction containing 2,6-dimethylnaphthalene.
2. Extraction: The pretreated fraction is extracted using a specific extractant to increase the concentration of 2,6-dimethylnaphthalene. This step is usually carried out in a liquid-liquid extraction column, where the separation of the components is achieved by selective dissolution of the solvent.
3. Vacuum distillation: The extracted solution is subjected to vacuum distillation to further enrich 2,6-dimethylnaphthalene and to remove some low-boiling impurities.
4. Melt crystallisation: the enriched solution is subjected to melt crystallisation under controlled conditions, taking advantage of the difference in melting points between 2,6-dimethylnaphthalene and other components to crystallise.
5. Separation and washing: The crystallised solid is separated by filtration or centrifugation and the crystals are washed to further remove impurities.
6. Refining: The washed crystals may undergo one or more recrystallisation processes to improve their purity.
8. Drying: Finally, the crystals are dried to remove residual solvent or mother liquor to obtain a high purity 2,6-dimethylnaphthalene product.
Specific process conditions, such as temperature, pressure, solvent type, operating time, etc., need to be optimised according to the actual situation. In practice, some special steps may also be included, such as the use of complexing agents to improve the separation efficiency of 2,6-dimethylnaphthalene, or the use of membrane separation technology to further purify the product.
In a study conducted by Ban et al. in 2019, the researchers first enriched the coal tar fraction to obtain 2,6-dimethylnaphthalene content of 11.56% by vacuum distillation, and then removed other isomeric impurities contained therein by melt crystallisation. Ultimately, they extracted dimethylnaphthalene with a purity of 62.64% and a yield of 68.12%. This process demonstrates how 2,6-dimethylnaphthalene can be efficiently extracted and purified from coal tar by coupling the techniques of vacuum distillation and melt crystallisation.
Phenazine, a valuable by-product in the production of 4-aminodiphenylamine, can be efficiently separated and purified by an extraction-coupled melt crystallisation process. The following is a specific process scheme:
1. Extraction: Firstly, 4-aminodiphenylamine production waste stream is passed through an extraction process using a suitable extractant to enrich phenazine. The purpose of this step is to increase the concentration of phenazine so that it can be easily separated and purified subsequently.
2. Melt crystallisation: The extracted solution is melt crystallised at a controlled temperature. Since the melting point of phenazine is different from that of the other components, the phenazine is preferentially crystallised by slow cooling.
3. Separation: The crystallised phenazine is separated from the solution by filtration or centrifugation.
4. Washing: The crystallised phenazine is washed to further remove impurities.
5. Drying: The washed phenazine crystals are dried under controlled conditions to remove residual solvent.
6. refinement: further crystallisation steps may be required to improve the purity of phenazine.
In a study by Li et al. in 2015, crude phenazine was separated from the waste stream by melt crystallisation, then the impurities in crude phenazine were extracted by extraction, and finally phenazine with purity up to 99.5% could be obtained by recrystallisation with a yield of about 85% , and the process flow is shown in the following figure. This method not only improves the purity and yield of phenazine, but also reduces environmental pollution and production costs.
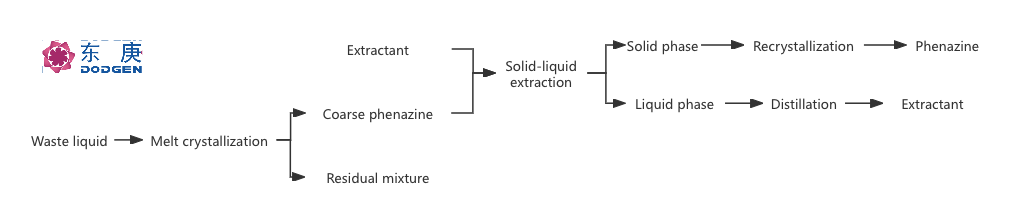
This extraction-coupled melt crystallisation process, by combining two different separation techniques, achieves the effective recovery and purification of phenazine from the waste stream of 4-aminodiphenylamine production, which is of great value for industrial application.
The coupled melt crystallisation and extraction process not only has the advantages of low energy consumption, short operation cycle and low pollution of the extraction process, but also has the advantages of high separation coefficient of melt crystallisation. The coupled process simplifies the process flow and improves the separation effect. DODGEN has the advanced technology of melt crystallisation and extraction, and can customise reasonable solutions for customers to achieve the ideal separation effect.
